Which Best Describes Trends in Electronegativity on the Periodic Table
Pogil worksheets trends in the periodic table worksheet answers 23 rustic worksheet periodic trends answers answer key pogil chemistry a study of matter table 4 periodic trends worksheet answers pogil worksheets periodic trends can the properties of an element be predicted using a table why is often considered to best friend. Thus the nonmetals which lie in the upper right tend to have the highest electronegativities with fluorine the most electronegative element of all EN 40.
Electronegativity Trend Science Trends
Note that some are not known such as atomic number 116.
. You give it atomic number and charge and it will give you the configuration one sub level at a time. The change in properties of elements down the groups from. The best guide to the covalent or ionic character of a bond is to consider the types of atoms involved and their relative positions in the periodic table.
The Periodic Table Questions and Answers. Since the electrophile can potentially be any atom on the periodic table except for H which would make nitrogen a base theres inherently a lot more variability possible for nucleophilicity than there is for basicity. Just as the weather changes during the entire year the properties of elements also change along the groups and periods in the Periodic table.
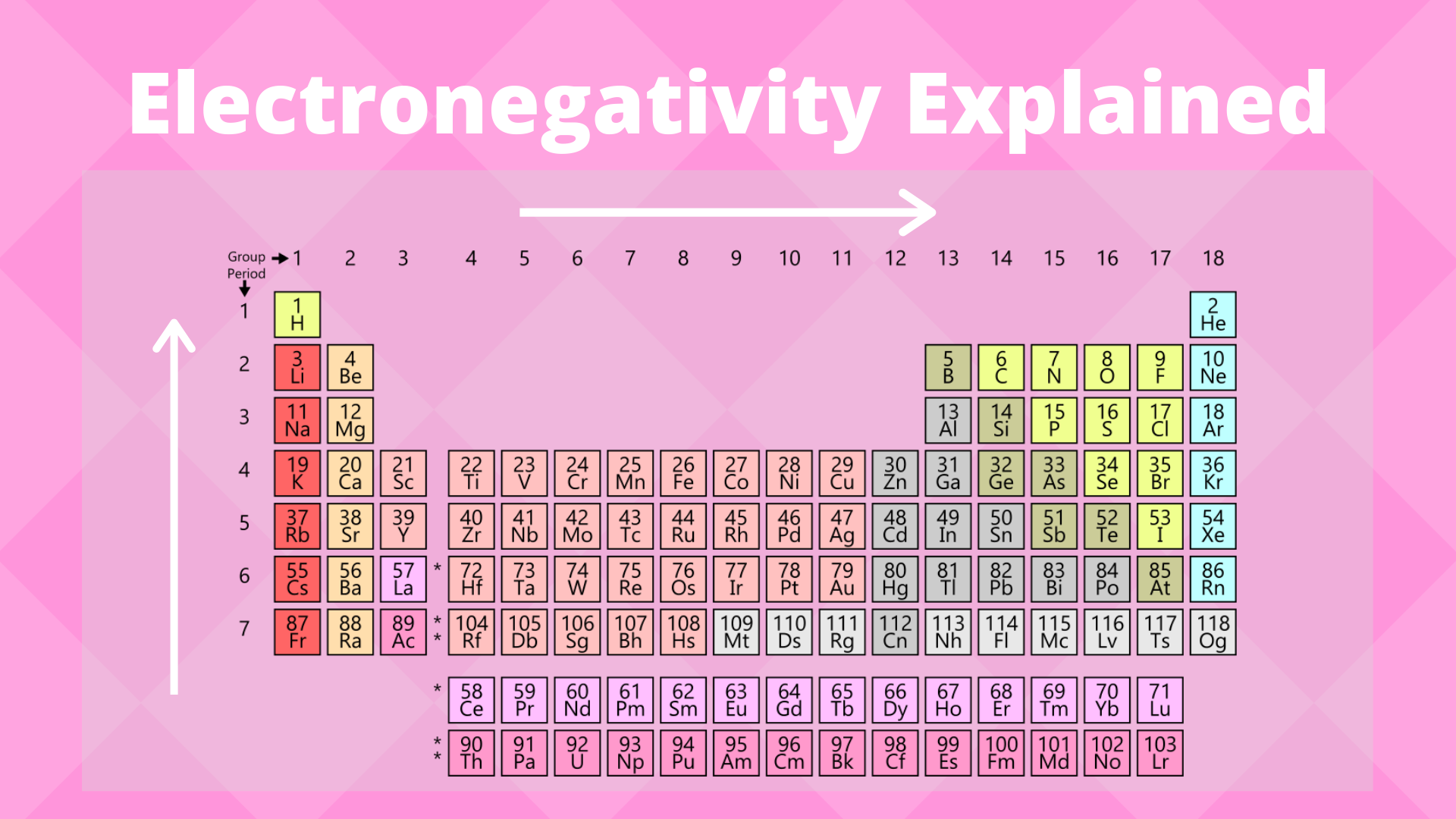
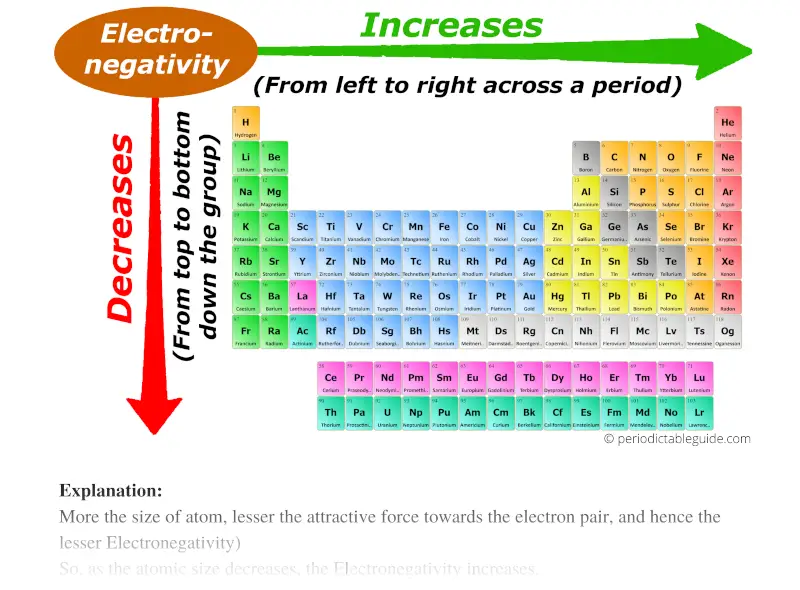
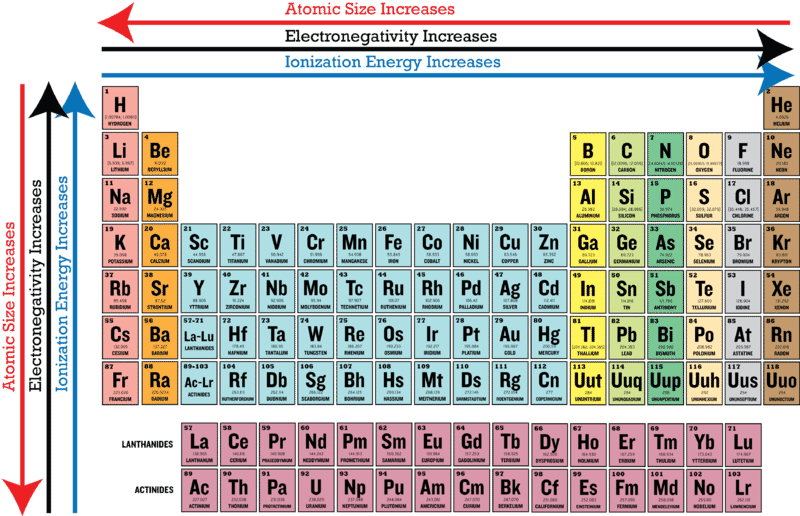
This information provides a coherent framework to analyze the. Periodic trends predict differences between elemental characteristics as you move across the periodic table. In general electronegativity increases from left to right across a period in the periodic table and decreases down a group.
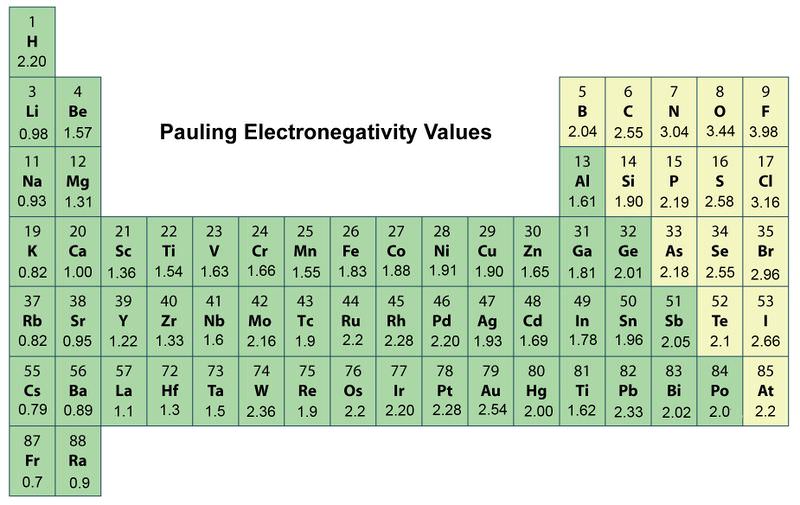
Fundamentals Of Experimental Design Pogil. Supplementary Materials contain our calculated electronegativities and chemical hardnesses of the elements as well as their electronic configurations magnetic moments and nonbonded volumes at different pressures. Electronegativity A periodic table modified to display the electronegativity for any element.
Metals tend to be less electronegative elements and the group 1 metals have the lowest. Periodic table of electronegativity at A 0 GPa B 200 GPa and C 500 GPa. As we move across a period from left to right the nuclear charge increases and the atomic size decreases therefore the value of electronegativity increases across a period in the modern periodic table.
For example the electronegativity trend across period 3 in the periodic table is depicted below. Electron Figures out the electron energy configuration. This page describes and explains the trends in atomic and physical properties of the Period 3 elements from sodium to argon.
Electronegativity is only defined for an atom as it describes the atoms tendency to attract shared electrons. On moving from left to right in the Periodic table electronegativity increases whereas on moving from top to bottom the electronegativity decreases. Here were going to confine ourselves largely to the reactions of amine bases with carbon-based electrophiles since its the most relevant for our.
Periodic Trends in the Electronegativities of Elements. Trends are based on Coulombs law which mathematically relates several characteristics of an elements. Probably the thing you were interested in calculating is the dipole moment of a.
Access the answers to hundreds of The periodic table questions that. 12 35 Study Notes on Unit 36 Dec 29 2021 Please send any. There are also some electronegativity trends that can be observed in the Periodic table.
Atomic size measured the distance between the nucleus of an atom and the outermost non-valence electrons of the atom. Get help with your The periodic table homework. 232 1 4 7 ANSWERS.
In the proper order too. Apart from this the electronegativity chart can also be created in accordance with the periodic table. You feel hot in summer and cold in winter.
The Periodic Table 3. Chapter 2 Worksheet problems answer key - CHEM 1311 - StuDocu Bio Chapter 2 Chem 67 Therefore hydrogen atoms have 1 proton and 1 electron carbon atoms have 6 protons and 6 electrons and oxygen atoms have 8 protons and 8 electrons. That means the climate or weather changes throughout the year starting from January to December.
It covers ionisation energy atomic radius electronegativity electrical conductivity melting point and boiling point. Likewise the Na and Cl atoms in NaCl have an electronegativity difference of 21 and the Mn and I atoms in MnI 2 have a difference of 10 yet both of these substances form ionic compounds.

All Periodic Trends In Periodic Table Explained With Image
Comments
Post a Comment